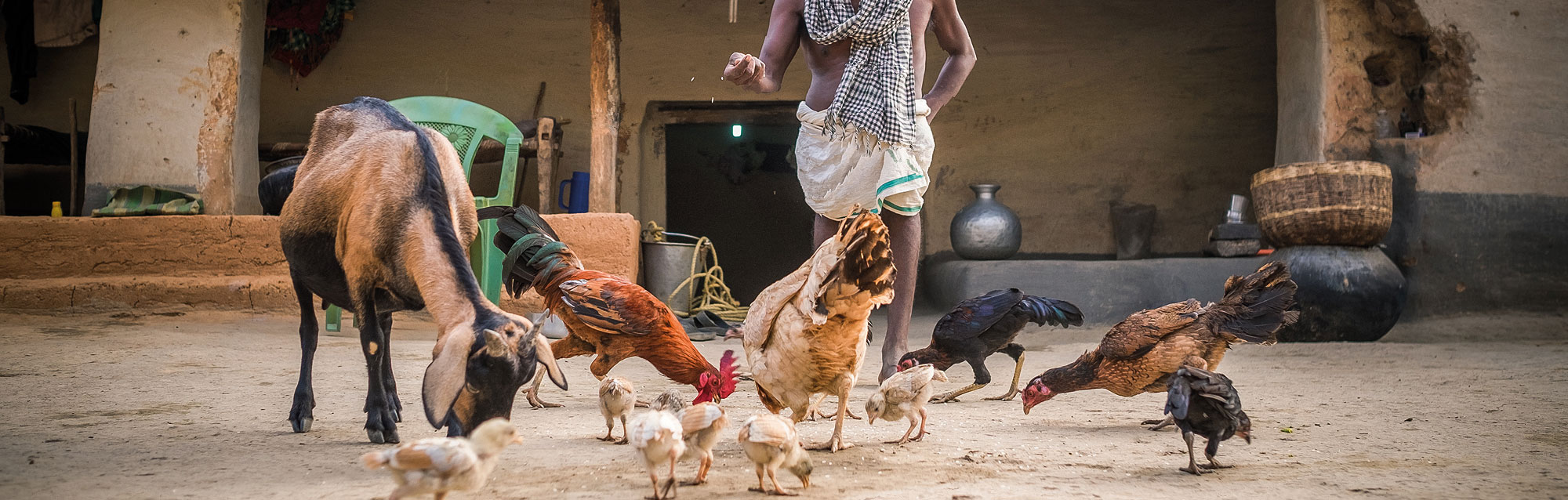In 2018, GALVmed marks 10 years of activities focused on bringing effective livestock vaccines, medicines and diagnostics to millions of smallholder farmers in sub-Saharan Africa and South Asia. Livestock are an intrinsic part of small-scale agriculture and of critical importance to the livelihoods of hundreds of millions of individuals and families. It is estimated that one in every five people depend on the livestock sector as a primary source of income. Livestock diseases therefore pose a significant risk to economic gains and livelihoods. At a household level, livestock are often the most valued possession for smallholder farmers. These valuable assets are also vulnerable assets: the mortality and morbidity rates of many livestock diseases in Africa and Asia are high. These diseases can lead to catastrophic loss for smallholder farmers.
GALVmed was created in 2008 from the recognition that while improved livestock health can create enormous benefits for smallholder farmers and can significantly improve productivity and livelihoods, suitable products and professional advice were not reaching smallholder farmers in developing countries. Effective livestock vaccines and medicines are valuable farming inputs that offer smallholder farmers, and donors, a return on investment that few other farming inputs can match. Our vision is to see such livestock vaccines and medicines in widespread, sustainable use by smallholder farmers.
-
2005
GALVmed formally incorporated with initial seed funding of GBP 2.6 million from the UK Government’s Department for International Development (DFID) to establish the organisation and seek long-term funding.
-
2008
GALVmed awarded USD 28 million joint funding from the Bill & Melinda Gates Foundation (BMGF) and DFID to implement the first Protecting Livestock, Saving Human Life programme (PLSHL 1).
-
2009
GALVmed awarded EUR 6.9 million from the European Commission via the African Union Inter-African Bureau for Animal Resources (AU-IBAR) to implement the Vaccines for the Control of Neglected Animal Diseases in Africa (VACNADA) programme aimed at improving the technical capabilities of eight national laboratories in the production of four key livestock vaccines.
-
2011
GALVmed awarded GBP 8 million from DFID to implement the first phase of the Animal African Trypanosomosis (Tryps) programme to develop a novel trypanocide.
-
2013
GALVmed awarded USD 52 million joint funding from BMGF and DFID to implement the second Protecting Livestock, Saving Human Life programme (PLSHL 2).
GALVmed awarded USD 1.5 million joint funding from BMGF and DFID via the Harbin Veterinary Research Institute to evaluate the Chinese-developed BEN-1 contagious bovine pleuropneumonia (CBPP) vaccine in Africa.
-
2014
GALVmed awarded USD 14.4 million by BMGF and DFID in a jointly funded programme to implement the second phase of the Tryps programme.
-
2016
GALVmed awarded GBP 200,000 from Innovate UK as part of a multidisciplinary collaboration to enhance the East Coast Fever Infection and Treatment Method (ECF-ITM) vaccine, particularly vaccine stability.
GALVmed awarded USD 1.7 million from AgResults, a multimillion dollar, multidonor, multilateral initiative, to implement the Brucellosis Vaccine Prize, a USD 30 million global competition to develop a vaccine against brucellosis.
-
2017
GALVmed awarded USD 50 million joint funding from BMGF and, subsequently in 2018, by DFID, to implement the Veterinary Innovations Transforming Animal Health & Livelihoods (VITAL) programme.
GALVmed awarded USD 3 million from BMGF to oversee and support a programme of activity to develop a viable business model for providing quality veterinary healthcare to smallholder farmers in Africa.
GALVmed awarded a further USD 4.3 million supplementary funding for the Tryps programme by BMGF and DFID.
Insight
Smallholders with larger flock sizes adopt vaccines earlier and at higher rates than those with smaller flock sizes.
ND vaccine uptake in Uganda (zero vaccine available at baseline)